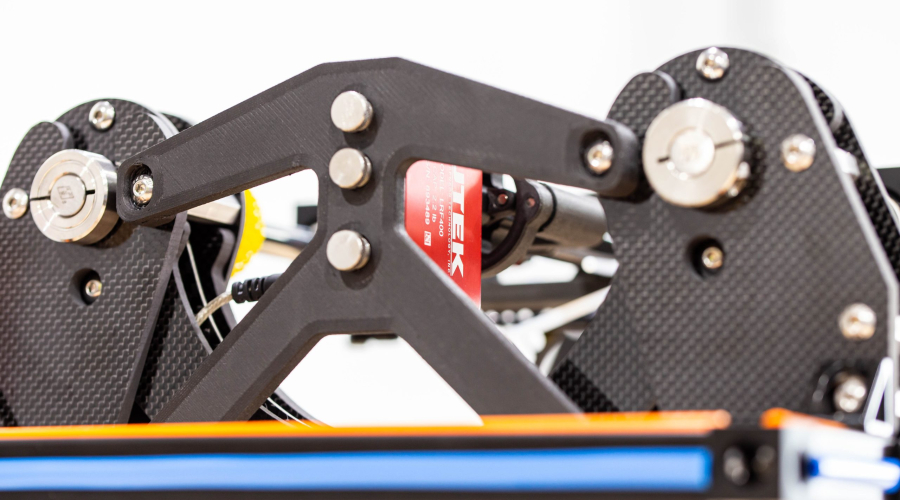
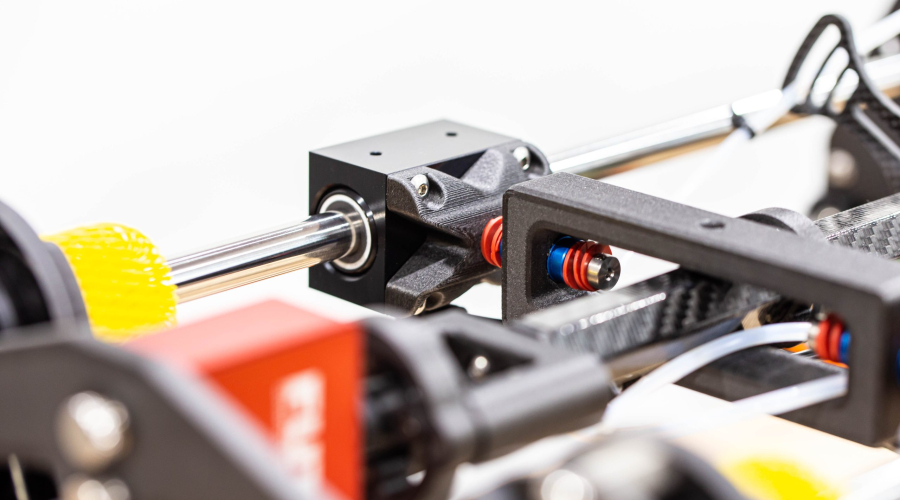
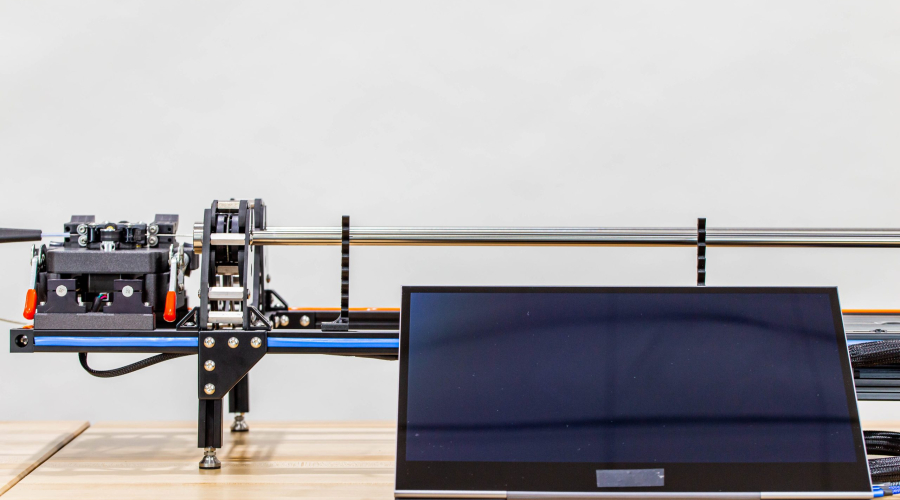
INSERTION FORCE TEST FIXTURE
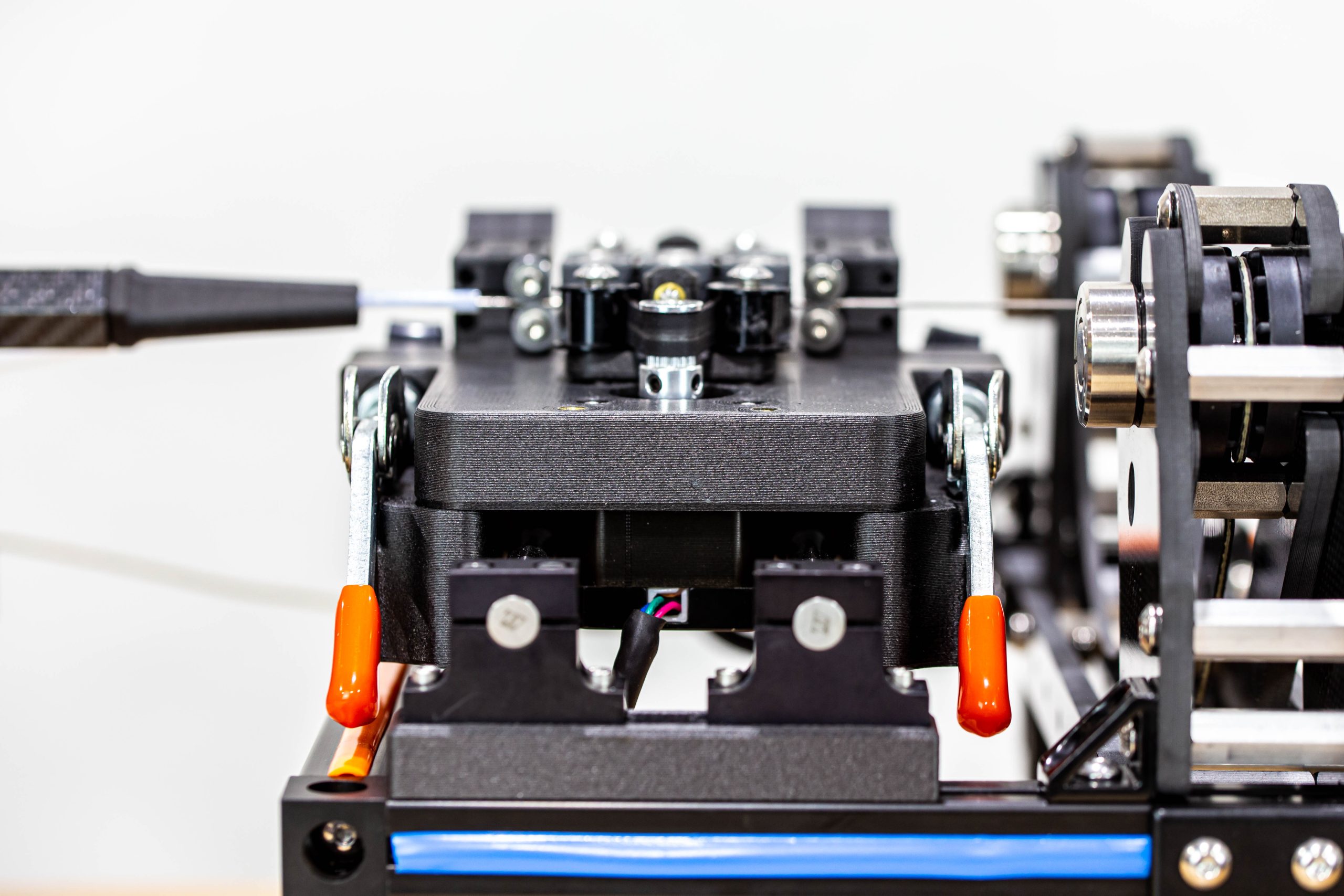
INSERTION FORCE TEST FIXTURE SUMMARY
Pipeline designed five fixtures to qualify the functionality of a medical device transseptal needle: Insertion Force, Tip Retention, Straightness, Shaft Cycling & Thermal Spread.
TEAM:
Mechanical Engineer, Controls Engineer, Software Engineer, Technician
DESIGN TOOLS USED:
Solidworks, 3D Printing, LabVIEW
MARKET:
Medical Devices
PROJECT REQUIREMENTS
- Fully automated measurement of insertion force of transseptal needle into phantom vasculature
- Fully automated measurement of straightness of transseptal needle
- Quantify thermal response of needle tip during use
- Monitoring and data acquisition of testing via software automation
SUMMARY OF ACTIVITIES
Pipeline’s customer needed a full suite of custom test fixtures to test and functionally qualify their device. In the images below, only the insertion force test fixture is shown. However, Pipeline also delivered an automated shaft straightness measuring test fixture, a tip retention test fixture, a shaft cycling test fixture, and a thermal measurement test fixture. Each test fixture was developed custom from the ground up and delivered as a turnkey solution specifically to meet the needs of this particular program.
Pipeline provided significant R&D efforts to determine acceptable processes and mechanisms to meet customer objectives. After the R&D phase was complete, Pipeline proceeded to complete detailed designs, drawings, procurement, software development, assembly, and functional verification of each fixture before delivering them to the customer.
The fixtures worked so well for the customer’s R&D activities that they asked Pipeline to develop a production version of the insertion force test fixture for qualifying the transseptal needle at their manufacturing facility. This practice of providing customers with R&D fixtures that then turn into production fixtures is common, and Pipeline is well equipped to support both R&D and production efforts.